
Our Program
To date, Olvi-Vec has been studied in multiple early- and mid-phase clinical trials via regional and systemic deliveries, as a monotherapy and in combination with other therapies, in approximately 150 patients suffering from a variety of cancer types.
Ovarian Cancer Program
Intraperitoneal (IPe) administration of Olvi-Vec is an attractive approach for high and condensed dosing in ovarian cancer.
Platinum-Resistant/Refractory Ovarian Cancer (PRROC)
PRROC represents a difficult-to-treat disease with a significant unmet medical need.
A Phase 2 clinical trial of Olvi-Vec in platinum-resistant/refractory ovarian cancer met the preestablished endpoints. The results showed that patients who received Olvi-Vec-primed immunochemotherapy demonstrated responsiveness to platinum-based therapy, to which they previously were deemed resistant or refractory. This was documented by multiple efficacy evaluation endpoints (based on pre-chemotherapy baseline), such as objective responses by ORR per RECIST 1.1 (54%) with durable response (7.6 months), median PFS (11.0 months) and median OS (15.7 months); of note, patients with platinum-refractory disease performed similarly well to platinum-resistant patients. Relative to historical comparisons, patients receiving Olvi-Vec-primed immunochemotherapy generally showed marked clinical benefits.
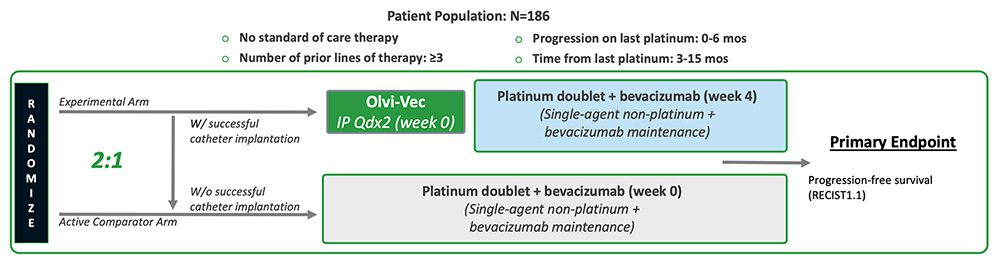
We are actively enrolling, OnPrime, our Phase 3 registration trial in PRROC (NCT05281471). The Phase 3 trial has an open-label, randomized controlled design (2:1 randomization), enrolling patients who have PRROC (disease progressed within six months since receiving last dose of or while on their last platinum regimen). The Experimental Arm patients will receive a single cycle (two doses) of Olvi-Vec administered intraperitoneally and, approximately four weeks later, a regimen of a platinum-based doublet plus bevacizumab followed by maintenance therapy. The Active Comparator Arm patients will receive a regimen of platinum-based doublet plus bevacizumab followed by maintenance therapy. The estimated enrollment is 186 patients. The following graphic summarizes the study design for the Phase 3 registration trial.
Systemic Delivery Program
The intravenous administration of Olvi-Vec is a potentially, appealing therapeutic approach that may impact the standard of care for many oncology patients because it allows for all tumors in a patient to be treated, including micro-metastases that are often difficult to detect and treat.
In a previously completed clinical study, Olvi-Vec, systemically administered as a monotherapy in patients with advanced solid tumors and with no standard of care option, showed a dose-dependent clinical benefit on overall survival (OS) from multiple intravenous cycles over months of time. The virus-dose-dependent OS benefit is even more noticeable in patients with intractable primary lung cancers and/or lung metastases of other tumor types.
Non-Small Cell Lung Cancer (NSCLC)
We are actively preparing to initiate a Phase 2 trial in NSCLC. Subject to regulatory approval, trial initiation is anticipated in the first quarter of 2023.
Despite advances in treatment and management of NSCLC, the disease represents a high unmet medical need. Novel therapies are required to improve therapeutic outcomes, especially in patients who do not have driver mutations for targeted therapies and/or immunotherapy, or who have developed resistance to their previous treatment(s).
V2ACT Pancreatic Cancer
V2ACT Therapeutics LLC, a joint venture with TVAX Biomedical to develop V2ACT Immunotherapy, holds an active IND and plans to conduct a Phase 1/2a trial for the treatment of newly diagnosed patients with surgically resectable pancreatic cancer.
Animal Health
A Phase 1 study of V-VET1 was completed in canine subjects with different tumor types, and evidence of antitumor responses and disease control was documented. V-VET1 is being developed by ELIAS Animal Health, our exclusive worldwide licensee.
Cancer is the leading cause of death for dogs and the number one pet health concern for dog owners in the United States. In addition to surgery, currently available canine cancer treatment typically provides limited survival benefit.
The National Cancer Institute’s Center for Cancer Research Comparative Oncology Program has reported that as many as six million pet dogs and six million pet cats are diagnosed with cancer annually in the United States. The veterinary oncology market is estimated to reach $909.4 million by 2026, with North America expected to hold a dominant position.




