
Product Candidates
Olvi-Vec, our lead product candidate, is a modified vaccinia virus that utilizes a triple mode of action: it directly kills cancer cells, stimulates a tumor-specific immune response and converts the tumor microenvironment from an immunosuppressive (cold state) to an immunoreactive (hot state).
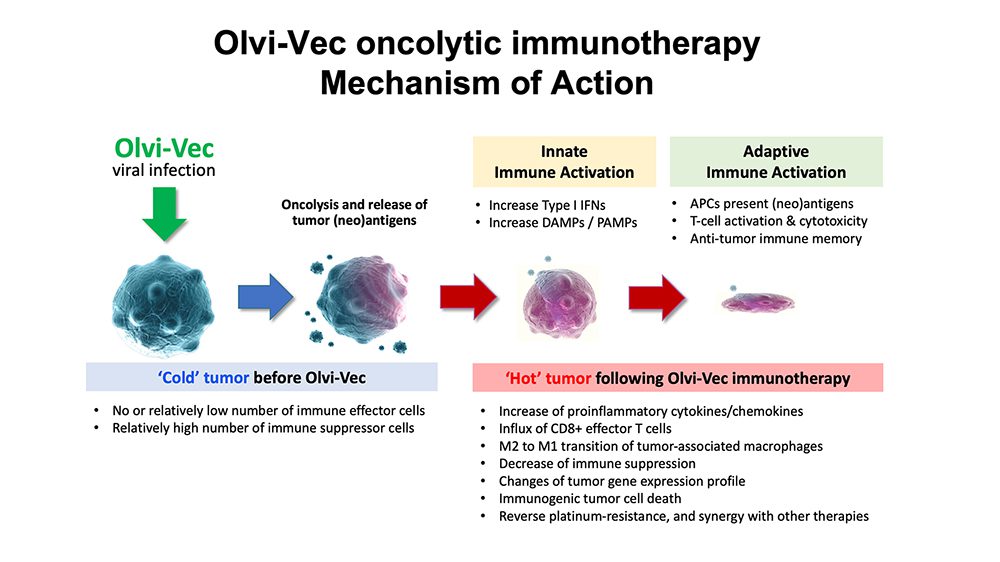
An elegant, differentiated, and highly desired immuno-oncology approach
-
- Physician-preferred/familiar methods of delivery locate and kill cancer cells to enhance antigen presentation and stimulate an anticancer immune response
Exciting Signals of Differentiated Therapeutic Potential
-
- Immunostimulatory backbone, by turning the tumor “hot”, with potential backbone for combination with other therapies, including chemotherapies
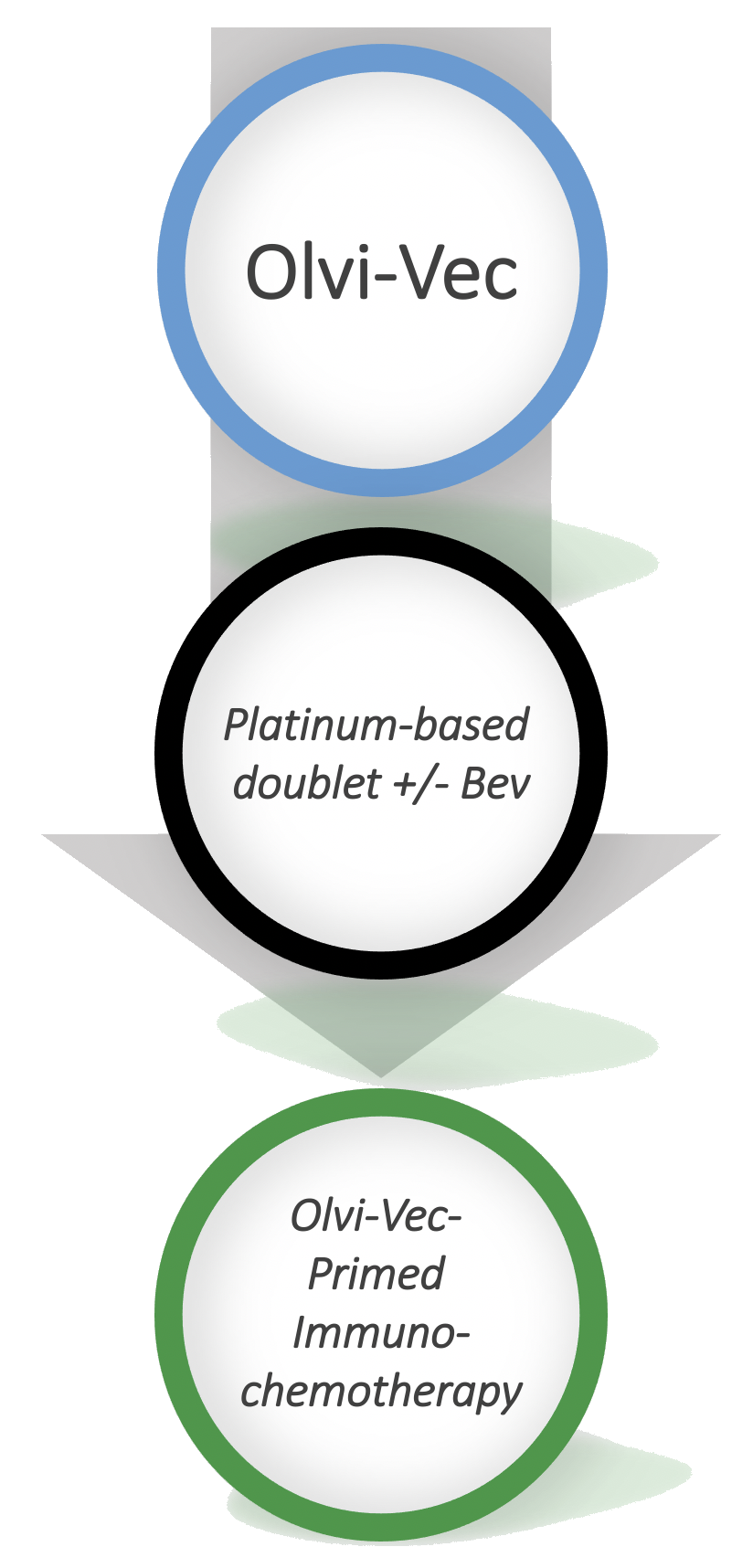
Oncolytic Vaccinia (Olvi-Vec) Primed Immunochemotherapy in Platinum-Resistant/ Refractory Ovarian Cancer
-
- Patients who received Olvi-Vec-primed immunochemotherapy demonstrated responsiveness to platinum-based therapy, to which they previously were deemed resistant or refractory.
- Annual Global Meeting of the International Gynecologic Cancer Society (IGCS) 2020 Oral Plenary Presentation on positive data of completed Phase 2 study: Link
We believe our pre-clinical and clinical data support the broad development of Olvi-Vec in patients with liquid or (metastatic) solid tumors, as a monotherapy or in combination with other therapies.
Our current plan is to expand our clinical development program by pursuing additional indications via intravenous delivery. Other indications will be selected from the balance of more than 20 major human cancers against which Olvi-Vec has shown activity in pre-clinical studies, including blood (leukemia/lymphoma), breast, cervical, colon, head & neck, kidney, lung, mesothelioma, ovarian, pancreatic, prostate and skin (melanoma) cancers.
V2ACT
Virus and Vaccine (Neoantigen)-Enhanced Adoptive Cell Therapy (V2ACT) Immunotherapy is a proprietary, indication-agnostic personalized anti-cancer designed to safely maximize the number and effect of cancer neoantigen-specific effector T cells within cancer tissues. It combines immunotherapeutic modalities, neoantigen-specific effector T cell immunotherapy (TVI) and oncolytic immunotherapy (initially, Olvi-Vec), each of which is supported by extensive preclinical and clinical proof-of-concept data, including Phase 1 and 2 clinical trials in various cancer indications. V2ACT Immunotherapy is being developed by V2ACT Therapeutics, LLC a joint venture in collaboration with TVAX Biomedical, Inc.
V2ACT: How it Works
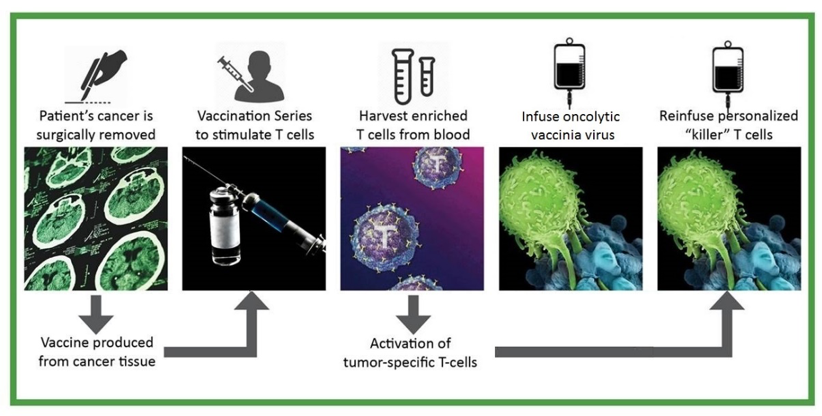
V2ACT: The Scientific Rationale
-
- Vaccinate a patient with the patient’s own neoantigen-containing cancer cells, combined with a powerful immunological adjuvant. Vaccination generates an immune response that produces high numbers of primed cancer neoantigen-specific effector T cell precursors in the patient’s body.
- Harvest immune cells from a vaccinated patient’s blood and stimulate with T cell activators ex vivo. Convert neoantigen-specific effector T cell precursors into effector T cells and increase their numbers.
- Treat the patient locally or systemically with Olvi-Vec. Olvi-Vec selectively enters cancer tissue and a) kills cancer cells, b) generates an immunostimulatory acute inflammatory response, a “hot spot” that increases receptivity to the anti-cancer effects of adoptively transferred neoantigen-specific effector T cells, and c) boosts anti-cancer immune responses.
- Infuse ex vivo-activated neoantigen-specific effector T cells into the patient. Effector T cells are carried to cancer tissue throughout the body, enter cancer tissue and initiate a cascade of immunological events that produce cancer cell killing.
- Treat the patient with a course of low-dose interleukin 2 (IL-2). IL-2 stimulates continued multiplication of infused cancer neoantigen-specific effector T cells.
V-VET1
V-VET1, an animal health product candidate, is a genetically characterized, veterinary-grade replication-competent oncolytic vaccinia virus that is a naturally attenuated isolate. It has been tested in a clinical study of canine cancers.




