In the journey through Platinum-Resistant or Platinum-Refractory Ovarian Cancer (PRROC), a promising area of focus is platinum re-sensitization. This innovative approach aims to re-sensitize cancer cells to platinum-based chemotherapy, which is the standard of care for ovarian cancer but becomes less effective once resistance develops. Platinum re-sensitization represents a spark of hope for those whose cancers have become resistant, potentially unlocking treatment options that were previously deemed ineffective.
Unmet Medical Need
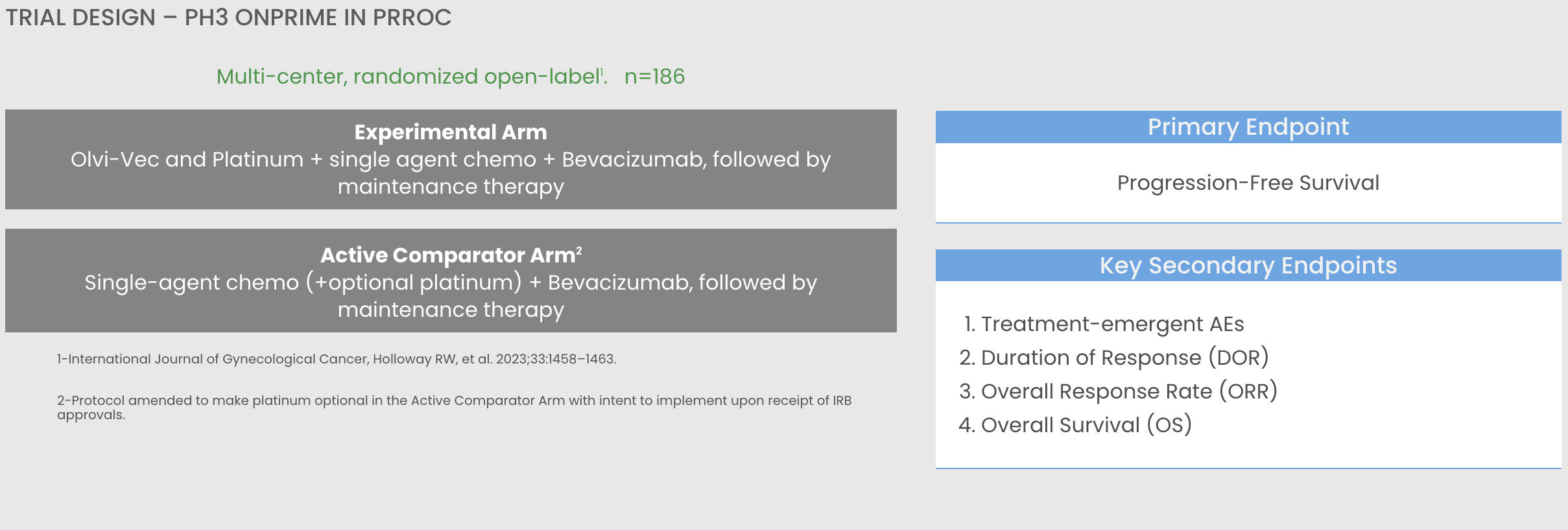
The development of novel, more effective therapies addresses a critical unmet medical need. Olvimulogene nanivacirepvec (Olvi-Vec), with its strong immune modulating effect on the tumor microenvironment, may provide re-sensitization to platinum and clinically reverse platinum-resistance or refractoriness in platinum-resistant/ refractory ovarian cancer. (International Journal of Gynecological Cancer Publication)
Phase 3 OnPrime Trial
REASONS FOR JOINING THIS PIVOTAL TRIAL
- No cap on the number of prior lines
- Primary & secondary platinum-refractory diseases also allowed
- No limitation on BRCA, HR or MMR status
- No requirement for tumor cell surface receptor or ligand
- Temporary catheter only in place for 1-2 weeks
- 2:1 chance of randomization into Experimental Arm

Do I Qualify for the OnPrime Trial?
New options are needed for late-stage ovarian cancer patients who failed prior treatments. Understanding the inclusion criteria is crucial to determine who can participate in our clinical trial.
NCT05281471 | Recruiting
Efficacy & Safety of Olvi-Vec and Platinum-doublet + Bevacizumab Compared to Physician’s Choice of Chemotherapy and Bevacizumab in Platinum-Resistant/Refractory Ovarian Cancer (OnPrime, GOG-3076)
Phase 3 | 18+ Years
Finding an OnPrime Clinical Trial Site
Navigating the complex landscape of Platinum-Resistant Refractory Ovarian Cancer (PRROC) involves exploring every possible avenue to optimize treatment and improve outcomes. One such avenue is participation in clinical trials, which can offer access to cutting-edge therapies and personalized approaches not yet available to the broader public.
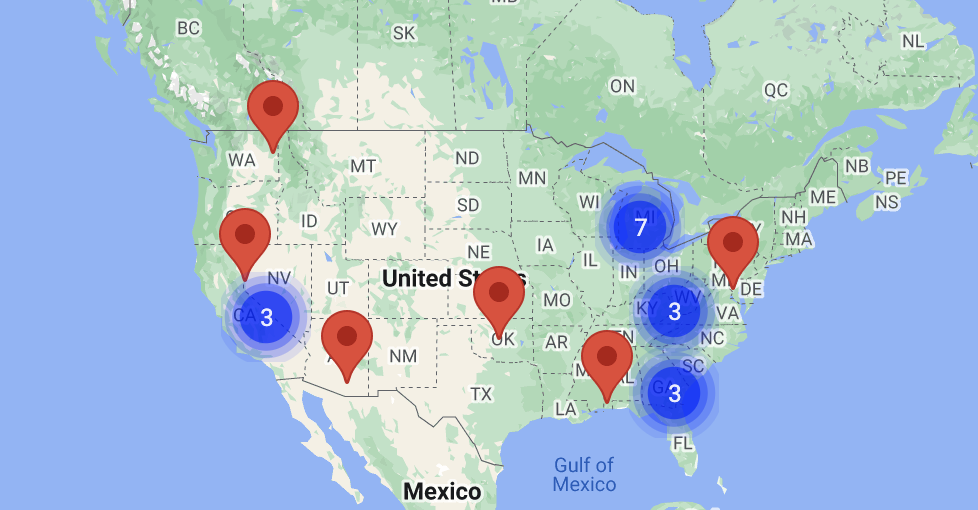
Locations in: AL, AZ, CA, FL, IN, MD, MI, MO, NV, NC, OH, OK, PA, SC, TN, WA
Clinical Trial Site Inquiries: Jane.LeBlanc@genelux.com
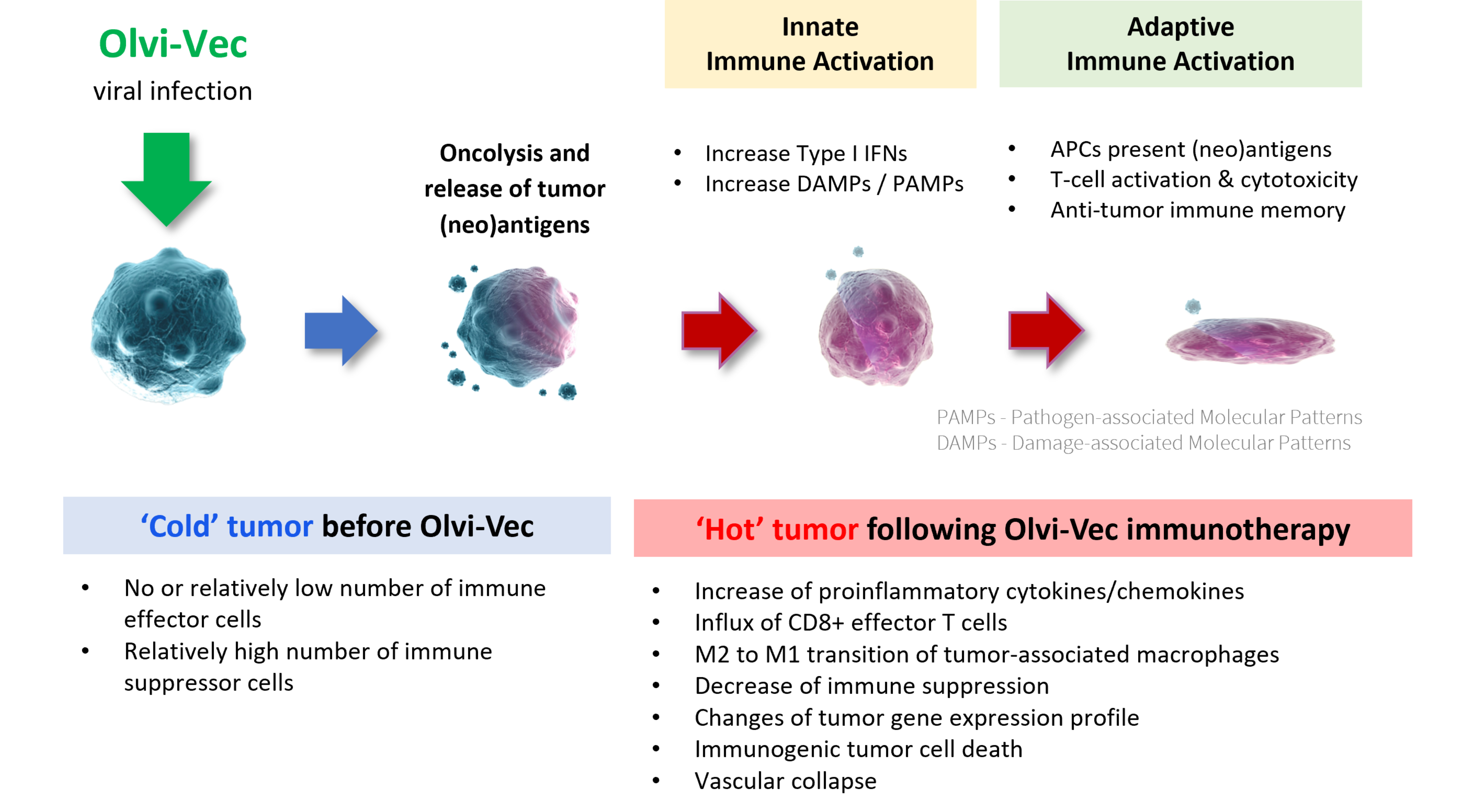
OLVI-VEC: How does it work?
Selective Replication In Tumors Unleashes Immune System Against Cancer
LEARN MORE
Key Takeaways
Olvi-Vec is a robust immune modulator that utilizes a triple mode of action to mount a personalized attack against cancer cells throughout the body
- Kills cancer cells directly, including cancer stem cells
- Enhances (neo)antigen presentation and stimulates a tumor-specific immune response
- Converts tumor microenvironment from immunosuppressive (cold state) to immunoreactive (hot state)
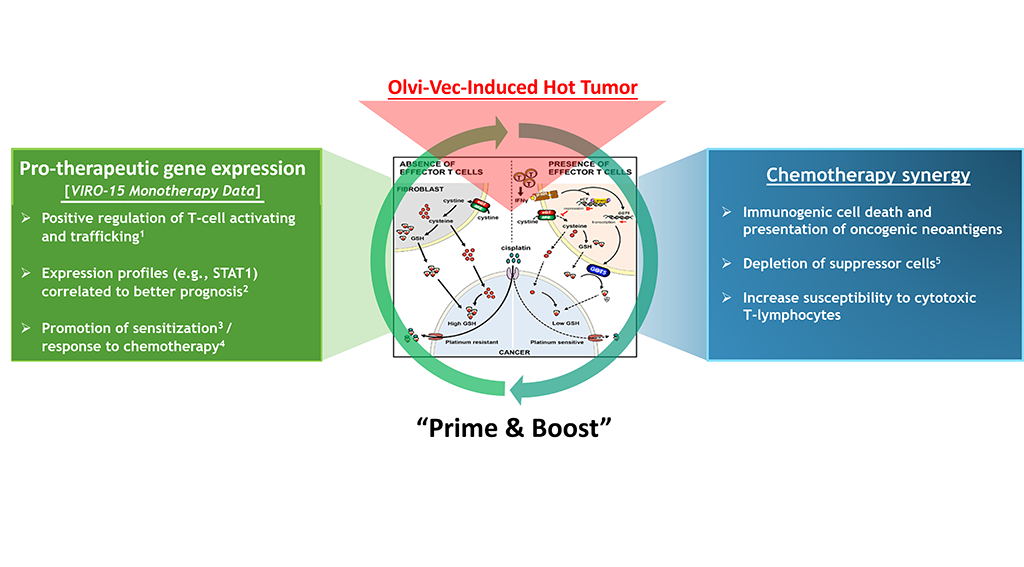
Olvi-Vec-Primed Immunochemotherapy: Reversing Platinum Resistance
LEARN MORE
Mechanisms on virus-mediated re-sensitization to platinum:
- Virus-induced immunogenic cell death (immune priming).
- Virus-induced intratumoral influx of CD8+ effector T cells, which abolish platinum resistance by altering glutathione and cystine metabolism in tumors.
- Virus-induced modulation of tumor gene expression to favor response to therapy.
- Cytotoxic chemotherapy induces immunogenic cell death (immune boosting), enhances antigen presentation, abrogates MDSC, Tregs.
1-Song et al. (Mol Ther (2007) 15(8):1558-1563)
2-Wang et al., Cell. 2016; 165(5): 1092–1105
3-Mantovani et al., J Exp Med. 2015;212(4):435–445
4-Ahmed et al., Mol Aspects Med. 2014;39:110-25
5-Weir et al., Cancers (Basel) (2011) 3(3):3114-3142 Emens et al., Cancer Immunol Res (2015) 3(5):436-443)
Patient Journey & Resources
Participating in a clinical trial is a significant step in a patient’s journey, especially for those facing Platinum-resistant or Platinum-refractory Ovarian Cancer (PRROC). This pathway offers not just the potential for personal treatment advancements but also contributes to the broader fight against ovarian cancer.
The journey through a clinical trial is a profound commitment by patients. It’s a path marked by hope, resilience, and the pursuit of new possibilities in the fight against PRROC.

Clinical Trial Information
Clinicaltrials.gov
Phase 3 Design
Phase 2 Results
General Cancer Organizations
National Cancer Institute
American Cancer Society
American Society of Clinical Oncology
Patient Advocacy Groups
Ovarian Cancer Research Alliance
Clearity Foundation
National Ovarian Cancer Coalition
Patient Voice
Frequently Asked Questions
Why join this pivotal trial?
- No cap on the number of prior lines of therapy
- Primary & secondary platinum-refractory diseases also allowed
- No limitation on BRCA, HR or MMR status
- No requirement for specific tumor cell surface receptor or ligand
- Temporary catheter only in place for 1-2 weeks
- 2:1 chance of randomization into Experimental Arm
What is the purpose of the Olvi-Vec-022 study?
The OnPrime trial is a Phase 3 registrational trial. The purpose of this research study is to determine how women diagnosed with platinum-resistant or platinum-refractory ovarian cancer will best respond to receiving Olvi-Vec followed by platinum- doublet chemotherapy (platinum-based chemotherapy such as carboplatin or cisplatin are given with a non-platinum based chemotherapy, including gemcitabine, paclitaxel, docetaxel, nab-paclitaxel, or pegylated liposomal doxorubicin [PLD]) along with bevacizumab, known as the Experimental Arm. If successful, the study is expected to support FDA approval.
As a phase 3 trial, this study is the last phase of testing to be completed before the drug is submitted for regulatory approval and as such a randomized design is required. The study is designed to confirm the tested drug effectiveness, safety and also compare it to standard treatment.
What is the investigational medication?
Olvi-Vec is a type of experimental treatment using a modified Vaccinia virus, designed to target and kill cancer cells without harming normal tissues. Vaccinia virus naturally does not cause disease in humans. Olvi-Vec works by entering cancer cells, breaking them down, and releasing new virus particles that help further attack the cancer. This process can also boost the body’s immune response against the tumor and generate other favorable anti-tumor effects.
Platinum is known to be the most effective therapy available for ovarian cancer patients, however most patients will eventually become resistant or refractory to treatment. In an earlier phase 2 trial (Holloway et al. JAMA Oncology, 2023; Holloway et al. Int J Gynecol Cancer, 2023), Olvi-Vec showed evidence of clinical reversal of platinum resistance or refractoriness in exemplary patients leading to potentially better outcomes then has been previously reported for this patient population.
Olvi-Vec is an experimental biologic and has not been approved by the U.S. Food and Drug Administration (FDA) and is not yet available for use outside of a clinical trial.
What is the study treatments and schedule?
If you are eligible and consent to participating in this study, you will be randomly assigned to receive one of two treatment arms. The experimental arm will have twice as many patients as the active comparator arm (2:1 ratio):
- Experimental Arm:
- Olvi-Vec – A total of 2 consecutive days of intraperitoneal catheter infusions in Week 0.
- A catheter is surgically placed into the abdominal cavity (also known as the peritoneal cavity) 2-7 days before the first Olvi-Vec infusion and removed within 2-7 days after the last Olvi-Vec infusion.
- Following catheter removal, you will receive platinum-doublet & bevacizumab (or biosimilar) administered as an intravenous infusion (IV) beginning in Week 4 or 5.
- Active Comparator Arm:
- Study treatment either with one of the non-platinum drugs and bevacizumab or with the platinum-doublet chemotherapy and bevacizumab (or biosimilar) beginning in the first week (Week 0).
If your condition is clinically stable, your disease does not significantly progress and you are tolerating the study treatment, you may continue to receive either the platinum doublet therapy plus bevacizumab or one of the non-platinum chemotherapy drugs and bevacizumab (or biosimilar).
How long will I be in the study?
After completing treatment and long-term follow-up, the total time on study is approximately 3 years.
What are the potential side effects of the Olvi-Vec treatment?
The following are the most commonly seen side effects from Olvi-Vec which usually are well managed with supportive care: gastrointestinal upsets such as nausea, vomiting and diarrhea; flu-like symptoms including fever, chills, cough, runny nose, sweating muscular aches, and pains; feeling tired/weak and headaches. Some women have also experienced abdominal pain and abdominal distension.
Will there be any cost to me if I participate in a clinical trial?
The experimental drug and any research-related procedures will be provided to you free of charge. You, or your insurance company, will be billed for those medications and treatments you would receive as the usual care for your disease. Please discuss expected costs with your study doctor.
You may be reimbursed for certain costs you acquire as a result of participating in this study. Your study team will provide you with the details, including amounts and any other requirements.
Whom to Contact
Jane LeBlanc
Director, Clinical Site Liaison
Genelux Corporation
Jane.LeBlanc@genelux.com
Main number: (805) 267-9889